Research & Development
The R&D practice area of BASE life science is dedicated to assisting pharmaceutical companies holistically and strategically. Our services encompass the broad scope of research and development. Our seasoned team combines in-depth knowledge of activities, regulatory requirements, and advanced digital solutions to deliver customized solutions tailored to meet the unique needs of our valued clients.
Within clinical activities, our consultancy excels at streamlining and optimizing the clinical trial process, ensuring efficient data collection and analysis, and facilitating collaboration among the different stakeholders. We offer regulatory support, assisting our customers in adhering to strict compliance guidelines and navigating the complex landscape of regulatory activities.
Embedded in our R&D portfolio, we leverage the Veeva value journey framework, amplifying the agility and impact of our clients’ operations. This involves the integration of Veeva solutions to streamline processes, improve data management, and enable seamless collaboration across various departments.
We offer a combination of deep pharmaceutical insights and tech expertise, crafting tailored digital solutions for the pharma industry. From building robust data infrastructures on leading cloud platforms to employing AI for actionable insights, our holistic approach addresses real-world challenges, ensuring precision and compliance at every step of the way.
R&D product and service offerings
We help you create business value from your cloud and data investments
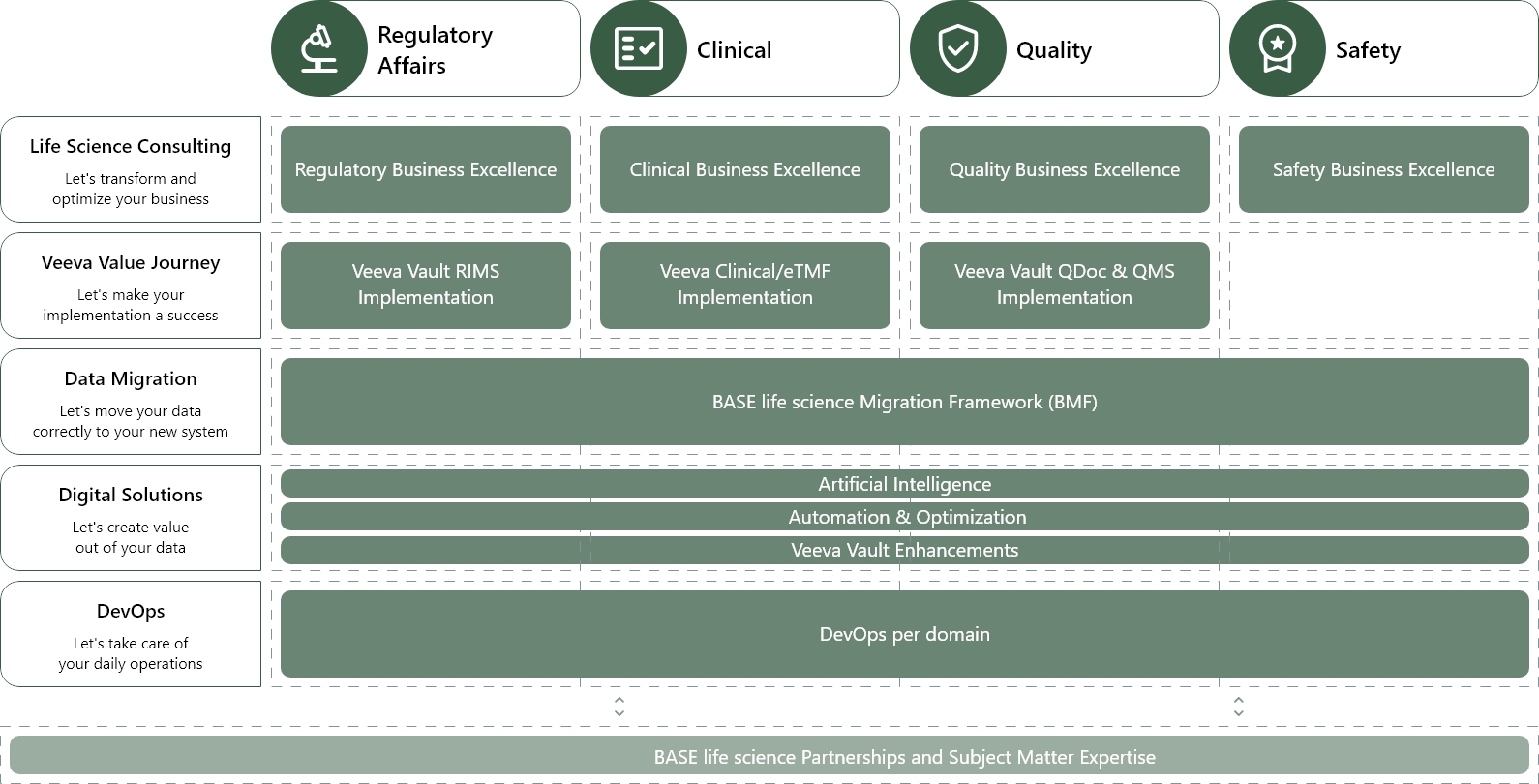
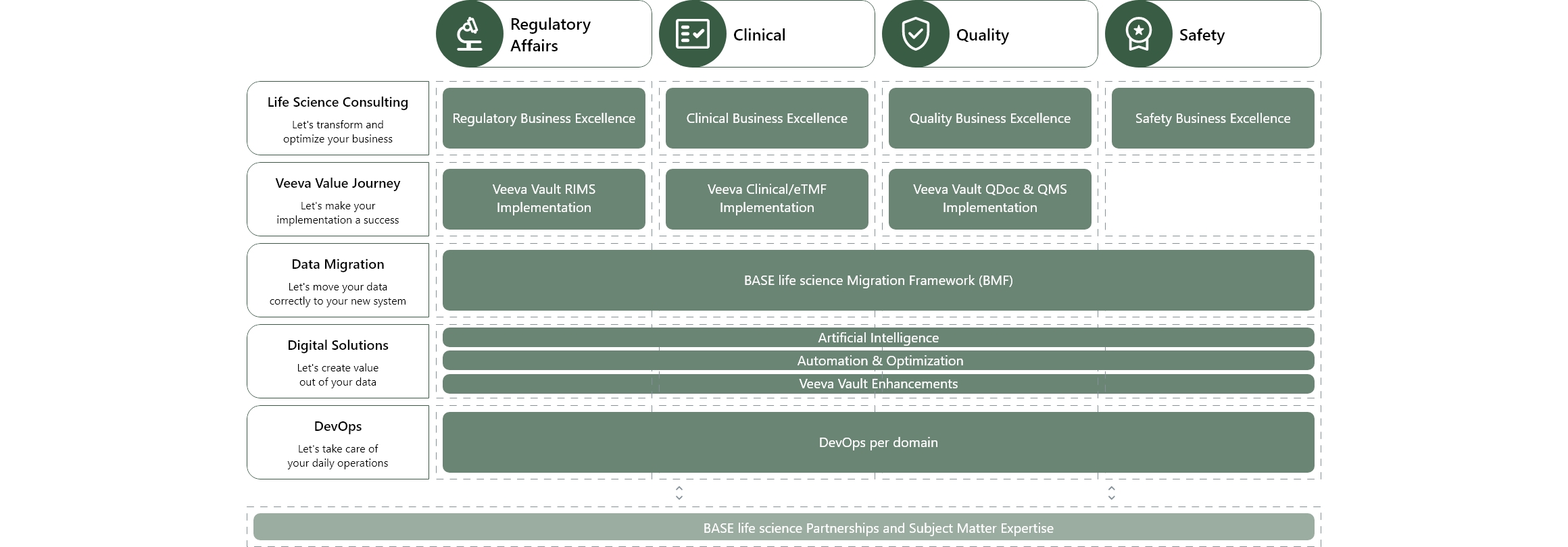
Our expertise spans program management, business and roadmap development, data migration, organizational change management, validation and testing, and integration. We proffer vast experience in managing complex projects, developing comprehensive blueprints in tandem with business objectives, seamlessly migrating data, overseeing organizational change, validating and testing systems, and ensuring seamless integration of your chosen IT solution.
In essence, our R& practice area combines deep domain expertise in the pharmaceutical industry with cutting-edge technological solutions, enabling our clients to foster innovation, ensure regulatory compliance, and achieve their research and development goals.
How our R&D offerings can benefit you
Certified Veeva Premium Service Partner
Dedicated Veeva Development Cloud experts throughout Europe since 2015
Life science process experts
Providing insights and awareness through in-depth analysis
Understanding technology, enabling a tangible difference
Redesigning processes to enable business to operate more effectively
Combining systems and processes
Bringing new technologies to the business based on accelerators and industry best practices

Veeva Development Cloud
We are part of a selected few globally to be certified Premiere partner in both Development and Commercial Cloud while also being a certified Veeva Migration Partner at the same time.
For Veeva Development Cloud we have delivered more than 70 services since 2007, spanning business processes and strategy to implementation, training and post-implementation support.
Our extensive experience cements us as an entrusted advisor, and you can safely place confidence in us and our abilities.
Read more
